Abstract
Introduction: Acalabrutinib (A) and ibrutinib (I) are both highly effective Bruton tyrosine kinase inhibitors (BTKi) approved for the treatment of chronic lymphocytic leukemia (CLL) and given as continuous treatments until disease progression or unacceptable toxicity. Venetoclax is a BH3 mimetic compound and B-cell lymphoma-2 inhibitor prescribed in combination with obinutuzumab (V+O) for a fixed (12-cycle) duration in treatment-naïve CLL patients. Although the ELEVATE-RR study demonstrated an improved safety profile of A compared with I in a head-to-head clinical trial, this study did not include treatment-naïve CLL patients (Byrd et al. J Clin Oncol. 2021). This MAIC builds upon the published analysis by Davids et al (Leuk Lymphoma. 2021) in treatment-naïve patients with CLL (which demonstrated a favorable safety profile for A-based therapy compared with other targeted therapies without compromising efficacy) by including longer follow-up data for A and the comparators.
Methods: Individual patient data for A ± obinutuzumab (A+O) from ELEVATE-TN (47 months median follow-up) (Sharman et al. ASCO 2021) were weighted to match the aggregate baseline characteristics of the I monotherapy arm from the ALLIANCE trial (Woyach et al. NEJM. 2018) (I + rituximab was not included as this treatment is not approved for CLL) and the V+O arm from the CLL-14 trial (Al-Sawaf et al. Lancet Oncol 2020). These baseline characteristics, TP53 mutation, serum β 2 microglobulin, ECOG, IGHV status, del(11q), CrCl, Rai stage or CLL-IPI, are potential prognostic variables (PV). Pseudo-individual patient data were generated from the digitized Kaplan-Meier curves published in the aforementioned comparator trials. An unanchored MAIC was conducted to adjust for these PVs between trials. The PVs selected were based on literature, clinical judgement, and demonstrated statistically significant association with progression-free survival (PFS) in univariate and multivariate regression analysis (Ahn et al. J Clin Oncol. 2020; Eichorst and Hallek. Hematol Am Soc Hematol Educ Prog. 2016). After matching, a weighted Cox proportional hazard model was used to analyze PFS and overall survival (OS) while a weighted logistic regression model was used for comparative safety analysis (grade ≥3 adverse events [AEs]). Two-sided p<0.05 was considered statistically significant.
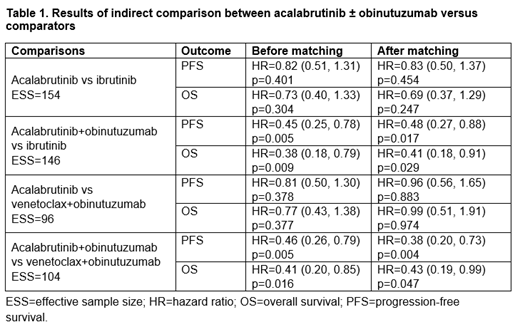
Results: This MAIC included 47-month data from ELEVATE-TN, 38-month data from ALLIANCE, and 40-month data from CLL-14 as opposed to 28-month data from ELEVATE-TN, 29-month data from RESONATE-2, and 29-month data from CLL-14 included in the previously published analysis. In the A vs I comparison, the PFS (hazard ratio [HR] 0.83 [95% CI 0.50, 1.37]) and OS (HR 0.69 [95% CI 0.37, 1.29]) numerically favored A but the difference was not significant. The A vs V+O comparison did not show significant differences in PFS (HR 0.96 [95% CI 0.56, 1.65] and OS (HR 0.99 [95% CI 0.51, 1.91]). For A+O vs I, significant differences in PFS (HR 0.48 [95% CI 0.27, 0.88]) and OS (HR 0.41 [95% CI 0.18, 0.91]) were observed. Similarly, for A+O vs V+O, significant differences were observed for PFS (HR 0.38 [95% CI 0.20, 0.73]) and OS (HR 0.43 [95% CI 0.19, 0.99]). Significant differences in rate of grade ≥3 AEs in favor of A and A+O were observed vs I for atrial fibrillation, hypertension, decreased neutrophil count, and decreased platelet count. Compared with V+O, patients treated with A had significantly lower rates of febrile neutropenia, leukopenia, neutropenia, thrombocytopenia, non-melanoma skin cancer, and secondary primary malignancies, excluding non-melanoma skin cancer. For A+O vs V+O, significantly lower rates of infusion-related reaction, neutropenia, and non-melanoma skin cancer were observed among patients treated with A+O.
Conclusions: Based on these MAIC results, A and A+O are associated with a favorable safety profile vs both I and V+O while, with longer follow-up, these MAIC results demonstrate that A+O is associated with a significant efficacy benefit vs both I and V+O. A limitation of this MAIC is not including all potential PVs as a trade-off to conserve the effective sample size. Our findings are consistent with the results of ELEVATE-RR comparing A with I in the relapsed population and also provide insight into comparisons of A-based therapy with V+O as we await more definitive prospective data on this question from a phase 3 trial.
Davids: Genentech: Consultancy, Research Funding; Ascentage Pharma: Consultancy, Research Funding; Surface Oncology: Research Funding; AbbVie: Consultancy; Adaptive Biotechnologies: Consultancy; BeiGene: Consultancy; Celgene: Consultancy; Eli Lilly and Company: Consultancy; BMS: Consultancy, Research Funding; MEI Pharma: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Verastem: Consultancy, Research Funding; Janssen: Consultancy; Novartis: Consultancy, Research Funding; Astra-Zeneca: Consultancy, Research Funding; MEI Pharma: Consultancy; Merck: Consultancy; Research to Practice: Consultancy; Takeda: Consultancy. Emeribe: AstraZeneca: Current Employment, Current holder of stock options in a privately-held company. Gaitonde: AstraZeneca: Current Employment, Current holder of stock options in a privately-held company, Research Funding. Cai: AstraZeneca: Current Employment, Current equity holder in publicly-traded company; Google: Current equity holder in publicly-traded company; Celgene Corporation: Ended employment in the past 24 months.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal